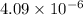Answer: The equilibrium constant for the total reaction is
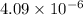
Step-by-step explanation:
We are given:
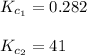
We are given two intermediate equations:
Equation 1:
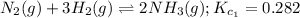
The expression of
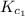 for the above equation is:
for the above equation is:
![K_(c_1)=([NH_3]^2)/([N_2][H_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/6iljzdl1c5p59p4zul0tnp8k7z9641fukq.png)
![0.282=([NH_3]^2)/([N_2][H_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/s1623v7auxnu6rmgigjhxuo9hl3reyc710.png) .......(1)
.......(1)
Equation 2:
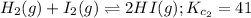
The expression of
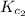 for the above equation is:
for the above equation is:
![K_(c_2)=([HI]^2)/([H_2][I_2])](https://img.qammunity.org/2020/formulas/chemistry/college/haco8ofnubgj0w076rip17rou2v33ycs6w.png)
![41=([HI]^2)/([H_2][I_2])](https://img.qammunity.org/2020/formulas/chemistry/college/plk4q9klks91hxltl1arj5u4m57dbufp8w.png) ......(2)
......(2)
Cubing both the sides of equation 2, because we need 3 moles of HI in the main expression if equilibrium constant.
![(41)^3=([HI]^6)/([H_2]^3[I_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/bwz5oe6o4y6e6a98fxn2xng5gm5td881mj.png)
Now, dividing expression 1 by expression 2, we get:
![(K_(c_1))/(K_(c_2))=\left((([NH_3]^2)/([N_2][H_2]^3))/(([HI]^6)/([H_2]^3[l_2]^3))\right)\\\\\\(0.282)/(68921)=([NH_3]^2[I_2]^3)/([N_2][HI]^6)](https://img.qammunity.org/2020/formulas/chemistry/college/5izq05xninnkkdsy74eivbw7vv4a38ijv3.png)
![([NH_3]^2[I_2]^3)/([N_2][HI]^6)=4.09* 10^(-6)](https://img.qammunity.org/2020/formulas/chemistry/college/qjf88n7n1jpdm7uh4ay6u61bq06hwnizuo.png)
The above expression is the expression for equilibrium constant of the total equation, which is:
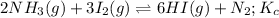
Hence, the equilibrium constant for the total reaction is