Answer:
volume of bubble is 2.736 cm³
Step-by-step explanation:
given data
height h = 35 m
volume = 1.98 cm³ = 1.98 ×
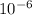 m³
m³
temperature t1 = 5.2°C
temperature t2 = 16.5°C
to find out
volume of the bubble
solution
first we find pressure depth 35 m
pressure = Pt + ρ × g × h ................1
here Pt is pressure at top = 1.013 ×
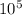 Pa and ρ density of water
Pa and ρ density of water
put here value in equation 1
pressure = Pt + ρ × g × h
pressure = 1.013 ×
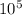 + 1000 × 9.8 × 35
+ 1000 × 9.8 × 35
pressure = 444300 Pa
so
from gas equation
p1×v1 / t1 = pt×v2 / t2
put here value
v2 = p1×v1×t1 / pt×t1
v2 = 444300× 1.98 ×
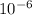 × 5.2 / ( 1.013 ×
× 5.2 / ( 1.013 ×
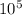 × 16.5 )
× 16.5 )
v2 = 2.736 ×
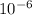 m³
m³
so volume of bubble is 2.736 cm³