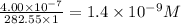Answer:
Molar concentration =
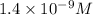
Step-by-step explanation:
Concentration of alkane (
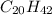 = 0.40 ppb
= 0.40 ppb
1 ppb =
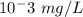
Concentration of alkane (
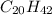 in mg/L
in mg/L
=
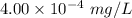
Concentration of alkane (
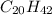 ) is
) is
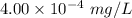 that means
that means
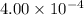 is present in 1 L
is present in 1 L
Density of water = 1.00 g/mL
Molar concentration =
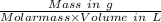
Formula weight = 282.55
Mass of
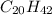 =
=
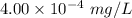 =
=
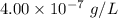
Here, formula mass is equal to molar mass.
Molar concentration =