Step-by-step explanation:
The given three gases are helium, nitrogen and propane.
It is known that
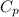 values of each of these gases is as follows.
values of each of these gases is as follows.
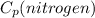 = 1.044 (KJ/Kg.K)
= 1.044 (KJ/Kg.K)
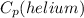 = 5.19 (KJ/Kg.K)
= 5.19 (KJ/Kg.K)
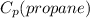 = 1.67 (KJ/Kg.K)
= 1.67 (KJ/Kg.K)
Now, relation between heat and
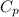 is as follows.
is as follows.
Q =
![n_(helium) * C_(p)(helium) * [T_(final) - 300]](https://img.qammunity.org/2020/formulas/chemistry/college/yyhk2gtl23ow6ca5y5w4zjt5q6n65t1l0o.png)
2 =
![(\rho_(helium) * V_(helium)) * C_(p)(helium) * [T_(final) - 300]](https://img.qammunity.org/2020/formulas/chemistry/college/1tcep8reubfvcxsqsp7yogbm2xqqp0c765.png)
2 =
![(0.179 * 0.005) * 5.19 [T_(final) - 300]](https://img.qammunity.org/2020/formulas/chemistry/college/8i21hsbpw2gmiou56pvzc7zsdrl57j32mk.png)
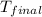 = 730.57 K
= 730.57 K
In the same way, Q =
![n_(nitrogen) * C_(p)(nitrogen) * [T_(final) - 300]](https://img.qammunity.org/2020/formulas/chemistry/college/i7g14ff0yunvxqgyg831cn40u7m76ka9rs.png)
2 =
![(1.2506 * 0.005) * 1.044 [T_(final) - 300]](https://img.qammunity.org/2020/formulas/chemistry/college/rtbsw4vrphs7hiq0xp5g7jlp7ju129zlnj.png)
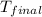 = 606.37 K
= 606.37 K
For propane also, Q =
![n_(propane) * C_(p)(propane) * [T_(final) - 300]](https://img.qammunity.org/2020/formulas/chemistry/college/wvlphsvoyzdtfs0nnhoskok2sv1lpdt91i.png)
2 =
![(1.4506 * 0.005) * 1.67 [T_(final) - 300]](https://img.qammunity.org/2020/formulas/chemistry/college/sjy3vh2u5z9uskf15psyuor2eh6ko8tw9u.png)
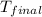 = 465.12 K
= 465.12 K
Here, we can see that there occurs least increase in temperature of propane whereas helium showed more increase in temperature.
This change arises because there density of helium is very less as compared to the density of propane and nitrogen. As a result, there occurs more turbulence in case of helium because of which it has less density and more rise in temperature.