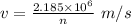Answer:
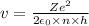
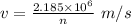
Step-by-step explanation:
According to Bohr's Theory,
The force of attraction acting between the electron and the nucleus is equal to the centrifugal force acting on the electron.
Thus,
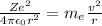 ......1
......1
Where, Z is the atomic number or the number of protons
r is the atomic radius
v is the velocity of the electron
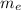 is the mass of the electron
is the mass of the electron
Also,
Accoriding to Bohr, the angular momentum is quantized. He states that the angular momemtum is equal to the integral multiple of
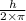 .
.
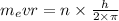 ....2
....2
solving r from equation 2, we get that:
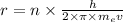
Putting in 1 , we get that:
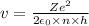
Applying values for hydrogen atom,
Z = 1
Mass of the electron (
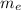 ) is 9.1093×10⁻³¹ kg
) is 9.1093×10⁻³¹ kg
Charge of electron (e) is 1.60217662 × 10⁻¹⁹ C
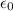 = 8.854×10⁻¹² C² N⁻¹ m⁻²
= 8.854×10⁻¹² C² N⁻¹ m⁻²
h is Plank's constant having value = 6.626×10⁻³⁴ m² kg / s
We get that: