Answer:
0.5286 kilogram of sulfur dioxide must be evaporated .
Step-by-step explanation:
Enthalpy of vaporization of chlorofluorocarbons
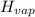 = 20.1 kJ/mol
= 20.1 kJ/mol
Mass of chlorofluorocarbons = 1.24 kg = 1240 g
Moles of chlorofluorocarbons =
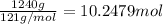
Heat removes by 10.2479 moles of chlorofluorocarbons : q
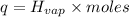
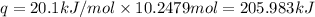
Let moles of sulfur dioxide removing 205.983 kJ(q) of heat be n.
Enthalpy of vaporization of sulfur dioxide
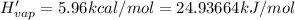
(1 kcal = 4.184 kJ)
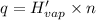
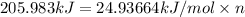
n = 8.2603 moles
Mass of 8.2603 moles of sulfur dioxide =
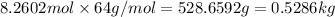
0.5286 kilogram of sulfur dioxide must be evaporated .