Answer:
Due to operator log it is impossible to reach a pH of 7 with these pKa's or acids used.
Step-by-step explanation:
Since the pKa values provided are not near to 7, reaching a neutral pH is not possible because of the governing equation equation of Buffer:
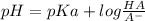
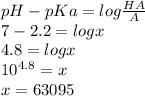
where x is\frac{HA}{A}
So the ratio of HA and A is vastly big so then it is almost impossible to arrive to this number.