Answer: The reaction proceed toward reactants because
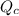 is 2.5
is 2.5
Step-by-step explanation:
 is the constant of a certain reaction at equilibrium while
is the constant of a certain reaction at equilibrium while
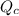 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
For the given chemical reaction:
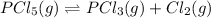
The expression of
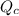 for above equation follows:
for above equation follows:
![Q_c=([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2020/formulas/chemistry/college/ny0hreedgnws5qqxb4xeu1pwk7tlxbo6mj.png)
We are given:
![[PCl_3]=0.20M](https://img.qammunity.org/2020/formulas/chemistry/college/k5p0gtlxvzrdrybw30fu90m6y4bxdmvhds.png)
![[Cl_2]=2.5M](https://img.qammunity.org/2020/formulas/chemistry/college/ewc6wbolbjlr7mt6z048dojefrn5756464.png)
![[PCl_5]=0.20M](https://img.qammunity.org/2020/formulas/chemistry/college/vuhq5tmm1opqpn86g1qm6c9zgixmr2ja4c.png)
Putting values in above equation, we get:
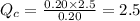
We are given:
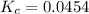
There are 3 conditions:
- When
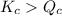 ; the reaction is product favored.
; the reaction is product favored. - When
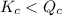 ; the reaction is reactant favored.
; the reaction is reactant favored. - When
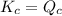 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
As,
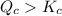 , the reaction will be favoring reactant side.
, the reaction will be favoring reactant side.
Hence, the reaction proceed toward reactants because
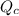 is 2.5
is 2.5