Answer:
1.
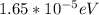 and
and
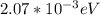 2.
2.
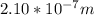
Step-by-step explanation:
The energy of a photon is given by the so-called Planck-Einstein relation:
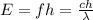
where E is the energy, f the frequency,
 the wavelength, c the speed of light and h the planck constant
the wavelength, c the speed of light and h the planck constant
So, we have:
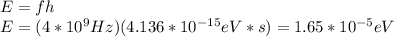
And:
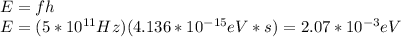
2. The work function is the amount of energy required to remove an electron from the material. Therefore the kinetic energy of the photoelectrons will be the energy of the electromagnetic radiation minus the work function:
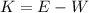
Where K is the kinetic energy and W the work function, for copper W=4.7eV
Rewriting for
 :
:
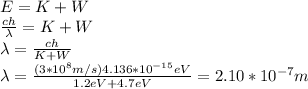
This wavelength corresponds to the ultraviolet range