Step-by-step explanation:
In order to find the molecular formula, we have to find the empirical formula first of all.
It is known that is a styrofoam, elements present are carbon and hydrogen atoms.
Element % Atomic mass Molar ratio Simple ratio
C 92.25 12.01
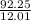 = 7.68
= 7.68
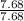 = 1
= 1
H 7.75 1.008
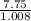 = 7.68
= 7.68
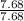 = 1
= 1
As empirical formula of styrofoam is C_{x}H_{x}.
Hence, empirical mass = (12.01 + 1.008) g/mol = 13.018 g/mol = 13.0 g/mol (approx)
Molar mass given is 104 g/mol.
So,
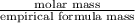 =
=
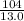 = 8
= 8
Thus, we can conclude that molecular formula of the given styrene is
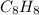 .
.