Step-by-step explanation:
According to law of conservation of mass, mass of the reactants is equal to the mass of products in a chemical equation. As mass can neither be created nor it can be destroyed but it can be transformed from one form to another.
As it is given that hydrogen and in excess oxygen is reacting that leads to the formation of water. Hence, the chemical reaction equation will be as follows.
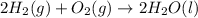
Since, it is given that 4 mol of hydrogen is reacting with excess of oxygen and gives 2 moles of water.
Hence, number of moles of water produced is calculated as follows.
4 mol of
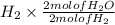
= 4 moles of
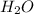
Thus, we can conclude that 4 moles of water you can produce from 4.0 mol of hydrogen and excess oxygen.