Answer:
17%
Step-by-step explanation:
Given parameters:
Atomic mass of nickel isotope = 57.9g/mol
Experimental value of atomic mass = 59.6g/mol
Unknown:
Percentage error in the measurement.
Solution:
The percentage error shows how much uncertainty was introduced into a measurement. To calculate this, we simply use the expression below:
Percentage error =
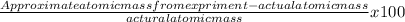
Percentage error =
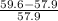 x 100 = 17%
x 100 = 17%