Step-by-step explanation:
It is known that 1 torr equals 1 mm Hg.
Therefore, 0.239 torr will be converted into mm Hg as follows.
1 torr = 1 mm Hg
So, 0.239 torr =
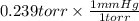
= 0.239 mm Hg
It means that 0.239 torr is equal to 0.239 mm Hg.
Therefore, we can conclude that partial pressure of given carbon dioxide in mm Hg is 0.239 mm Hg.