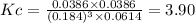Answer:
Kc = 3.90
Step-by-step explanation:
CO reacts with
 to form
to form
 and
and
 . balanced reaction is:
. balanced reaction is:
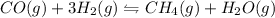
No. of moles of CO = 0.800 mol
No. of moles of
 = 2.40 mol
= 2.40 mol
Volume = 8.00 L
Concentration =
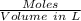
Concentration of CO =
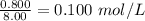
Concentration of
 =
=
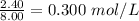
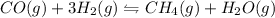
Initial 0.100 0.300 0 0
equi. 0.100 -x 0.300 - 3x x x
It is given that,
at equilibrium
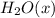 = 0.309/8.00 = 0.0386 M
= 0.309/8.00 = 0.0386 M
So, at equilibrium CO = 0.100 - 0.0386 = 0.0614 M
At equilibrium
 = 0.300 - 0.0386 × 3 = 0.184 M
= 0.300 - 0.0386 × 3 = 0.184 M
At equilibrium
 = 0.0386 M
= 0.0386 M
![Kc=([H_2O][CH_4])/([CO][H_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/lmbmjowihrshg39pmbuintl4z6qqrlv1dt.png)