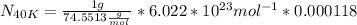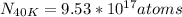Answer:
 atoms of 40K
atoms of 40K
Step-by-step explanation:
You can use the molecular mass and the Avogadro´s number, in the following formula:
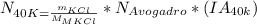
where
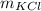 is the sample mass,
is the sample mass,
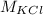 is the molecular mass of the KCl and IA(40K) is the isotopic abundance of 40K.
is the molecular mass of the KCl and IA(40K) is the isotopic abundance of 40K.
Now replacing the values, you can find: