Answer : The correct option is, (D) 3600 kJ
Explanation :
Mass of octane = 75 g
Molar mass of octane = 114.23 g/mole
Enthalpy of combustion = -5500 kJ/mol
First we have to calculate the moles of octane.
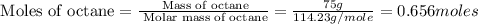
Now we have to calculate the heat released in the reaction.
As, 1 mole of octane released heat = -5500 kJ
So, 0.656 mole of octane released heat = 0.656 × (-5500 kJ)
= -3608 kJ
≈ -3600 kJ
Therefore, the heat released in the reaction is 3600 kJ