Answer:
total amount of fluoride 3.6 *10^6 g
Step-by-step explanation:
we know that
volume of reservoir
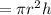
=
![\pi* [(4.70*10^2)/(2)]^2 *14.2](https://img.qammunity.org/2020/formulas/physics/high-school/grv0fil47d5lcpgco4cs0sidf1cdd0z5tb.png)
= 2.25*10^6 m^3
denso=ity of water = 1000 kg/m3
mass of water = 1000*2.25*10^6
= 2.25*10^9 kg = 2.25*10^12 g
fluoride 1.6 ppm
total amount of fluoride
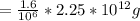
= 3.6 *10^6 g