Step-by-step explanation:
pH is the negative logarithm of hydronium ion concentration present in a solution.
- If the solution has high hydrogen ion concentration, then the pH will be low and the solution will be acidic. The pH range of acidic solution is 0 to 6.9
- If the solution has low hydrogen ion concentration, then the pH will be high and the solution will be basic. The pH range of basic solution is 7.1 to 14
- The solution having pH equal to 7 is termed as neutral solution.
To calculate the pH of the solution, we use equation:
![pH=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/f390cegazdnm7uy3e4lyqajx4gquacwg62.png) ......(1)
......(1)
To calculate the pOH of the solution, we use the equation:
pH + pOH = 14 ........(2)
We are given:
pH = 5.54
Putting values in equation 1, we get:
![5.54=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/syozu94dphm1c0k6g0yzg1232ftgplez4f.png)
![[H_3O^+]=2.88* 10^(-6)M](https://img.qammunity.org/2020/formulas/chemistry/college/zsnlrmp4kthmu3kymvbjnvr6085l6b69hl.png)
Now, putting values in equation 2, we get:
14 = 5.54 + pOH
pOH = 8.46
The solution is acidic in nature.
We are given:
pOH = 9.7
Putting values in equation 2, we get:
14 = 9.7 + pH
pH = 4.3
Now, putting values in equation 1, we get:
![4.3=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/wgwwbu8exwxayx0vyfil27mpjj4rczg2i1.png)
![[H_3O^+]=5.012* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/college/7jm8jk0zmhezdbjkck73r0mnrtd83nfawh.png)
The solution is acidic in nature.
We are given:
pH = 7.0
Putting values in equation 1, we get:
![7.0=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/n9sjqxpf3w8eejcseqteqeo1df6uvxo0da.png)
![[H_3O^+]=1.00* 10^(-7)M](https://img.qammunity.org/2020/formulas/chemistry/college/jr92wykfs51l0ssk84sef1v3gr4bsxiaj5.png)
Now, putting values in equation 2, we get:
14 = 7.0 + pOH
pOH = 7.0
The solution is neither acidic nor basic in nature.
We are given:
pH = 12.9
Putting values in equation 1, we get:
![12.9=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/9t4zt8xdzpbpdhmjdijgrkceki17rlvq1w.png)
![[H_3O^+]=1.26* 10^(-13)M](https://img.qammunity.org/2020/formulas/chemistry/college/3h8x7s5la91rycqqm8ca2nvam8rxt409ok.png)
Now, putting values in equation 2, we get:
14 = 12.9 + pOH
pOH = 1.1
The solution is basic in nature.
We are given:
pOH = 1.2
Putting values in equation 2, we get:
14 = 1.2 + pH
pH = 12.8
Now, putting values in equation 1, we get:
![12.8=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/f6uxbkhni6gerc03aji6p7dvyl12gvscoe.png)
![[H_3O^+]=1.58* 10^(-13)M](https://img.qammunity.org/2020/formulas/chemistry/college/23ksotiwlwxr31qlgezblkp0aoo794f8ne.png)
The solution is basic in nature.
We are given:
![[H_3O^+]=1* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/college/3z77lo3gfoo24odqml5003rioky4n29x8o.png)
Putting values in equation 1, we get:
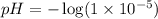
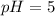
Now, putting values in equation 2, we get:
14 = 5 + pOH
pOH = 9
The solution is acidic in nature.