Answer:
Limiting reagent = lead(II) acetate
Theoretical yield = 1.2704 g
% yield = 78.09 %
Step-by-step explanation:
Considering:
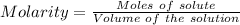
Or,
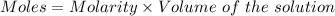
Given :
For potassium sulfate :
Molarity = 0.120 M
Volume = 57.0 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 57.0×10⁻³ L
Thus, moles of potassium sulfate:
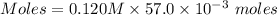
Moles of potassium sulfate = 0.00684 moles
For lead(II) acetate :
Molarity = 0.118 M
Volume = 35.5 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 35.5×10⁻³ L
Thus, moles of lead(II) acetate :
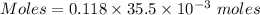
Moles of lead(II) acetate = 0.004189 moles
According to the given reaction:
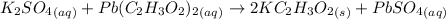
1 mole of potassium sulfate react with 1 mole of lead(II) acetate
0.00684 moles potassium sulfate react with 0.00684 mole of lead(II) acetate
Moles of lead(II) acetate = 0.004189 moles
Limiting reagent is the one which is present in small amount. Thus, lead(II) acetate is limiting reagent. ( 0.004189 < 0.00684)
The formation of the product is governed by the limiting reagent. So,
1 mole of lead(II) acetate gives 1 mole of lead(II) sulfate
0.004189 mole of lead(II) acetate gives 0.004189 mole of lead(II) sulfate
Molar mass of lead(II) sulfate = 303.26 g/mol
Mass of lead(II) sulfate = Moles × Molar mass = 0.004189 × 303.26 g = 1.2704 g
Theoretical yield = 1.2704 g
Given experimental yield = 0.992 g
% yield = (Experimental yield / Theoretical yield) × 100 = (0.992/1.2704 g) × 100 = 78.09 %