Answer: The concentration of hydrochloric acid in the final solution is 0.72 M
Step-by-step explanation:
According to the dilution law,

where,
 = molarity of stock solution = 3.95 M
= molarity of stock solution = 3.95 M
 = volume of stock solution = 268 ml
= volume of stock solution = 268 ml
 = molarity of diluted solution = ?
= molarity of diluted solution = ?
 = volume of diluted solution = 803 ml
= volume of diluted solution = 803 ml
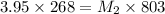
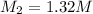
Next, you take 100.00 ml of that solution and dilute it to 184 ml in a volumetric flask.
According to the dilution law,

where,
 = molarity of stock solution = 1.32 M
= molarity of stock solution = 1.32 M
 = volume of stock solution = 100 ml
= volume of stock solution = 100 ml
 = molarity of diluted solution = ?
= molarity of diluted solution = ?
 = volume of diluted solution = 184 ml
= volume of diluted solution = 184 ml
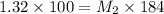
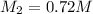
Thus concentration of hydrochloric acid in the final solution is 0.72 M