Answer: The volume of NaOH that must be added is 32.3 mL
Step-by-step explanation:
Let us assume that volume of NaOH required is 'V' mL
To calculate the millimoles of NaOH, we use the equation:
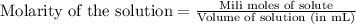
Molarity of NaOH solution = 0.1 M
Volume of solution = V mL
Putting values in above equation, we get:
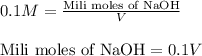
Molarity of HCOOH solution = 0.1 M
Volume of solution = 50 mL
Putting values in above equation, we get:
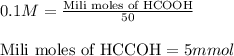
The chemical equation for the reaction of formic acid and sodium hydroxide follows:

Initial: 5 0.1V
Final: 5 - 0.1 V - 0.1V -
- To calculate the
 of acid, we use the equation:
of acid, we use the equation:
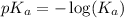
where,
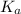 = acid dissociation constant =
= acid dissociation constant =
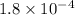
Putting values in above equation, we get:
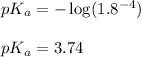
- To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2020/formulas/chemistry/college/wwf6o5cvurukvvigp9qetx7pcmu718wast.png)
![pH=pK_a+\log(([HCOONa])/([HCOOH]))](https://img.qammunity.org/2020/formulas/chemistry/college/4loqdahwk4yfxzkwmek359ncocg7sho5wc.png)
We are given:
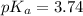
[HCOONa] = 0.1V
[HCOOH] = 5 - 0.1V
pH = 4.0
Putting values in above equation, we get:
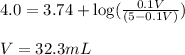
Hence, the volume of NaOH that must be added is 32.3 mL