Answer: The equilibrium constant for the reaction at 25 °C is 346.7
Step-by-step explanation:
Formula used :
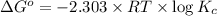
where,
 = standard Gibb's free energy change = -14.50kJ/mol =14500 J/mol
= standard Gibb's free energy change = -14.50kJ/mol =14500 J/mol
R = universal gas constant = 8.314 J/K/mole
T = temperature =
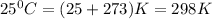
 = equilibrium constant = ?
= equilibrium constant = ?
Putting in the values we get:
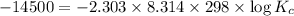
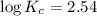
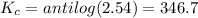
The equilibrium constant for the reaction at 25 °C is 346.7