Answer:
Option D) 2.89.
Step-by-step explanation:
Look up the acid dissociation constant of acetic acid:
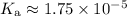
(CRC Handbook of Chemistry and Physics, 84th Edition (2004).)
Acetic acid partially dissociate to produce acetate ions and hydrogen ions:
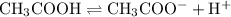 .
.
Let the final concentration of
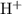 in the solution be
in the solution be
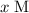 . The concentration of acetic acid would have dropped by
. The concentration of acetic acid would have dropped by
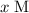 and the concentration of acetate ions would have increased by
and the concentration of acetate ions would have increased by
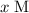 . The initial concentration of
. The initial concentration of
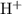 in pure water is
in pure water is
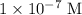 and will barely influence the outcome.
and will barely influence the outcome.
Construct a RICE table for this reaction: (all values here are in
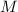 , which stands for concentrations in moles per liter.)
, which stands for concentrations in moles per liter.)
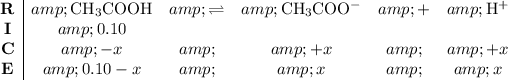 .
.
At equilibrium:
By the definition of the acid dissociation constant,
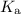 :
:
![\displaystyle K_(\rm a)(\mathrm{CH_3COOH}) = \frac{[\mathrm{CH_3COO^(-)}]\cdot [\mathrm{H^(+)}]}{[\mathrm{CH_3COOH}]}](https://img.qammunity.org/2020/formulas/chemistry/college/wdi8w5g87hkgsqlcl257gdybjwoepymo00.png) .
.
That is:
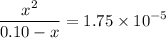 .
.
Rearrange and solve for
 :
:
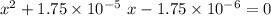 .
.
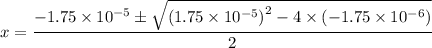 .
.
There might be more than one solution to this equation. However, keep in mind that all concentration should be positive (at least non-negative.) The only possible value of
 will thus be approximately
will thus be approximately
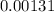 .
.
In other words, at equilibrium
![[\mathrm{H^(+)}] \approx 0.00131 \; \rm M](https://img.qammunity.org/2020/formulas/chemistry/college/afnqbk0amvuza1wato4qe2yw42c2xft13m.png) . By the definition of pH,
. By the definition of pH,
![\begin{aligned} \rm pH &= -\log_(10){[\mathrm{H^(+)]}\\&= - log_10(0.00131) \\&\approx 2.9\end{aligned}](https://img.qammunity.org/2020/formulas/chemistry/college/d7vb4l0pna3t1irwms2ckfbc9yralhnlxp.png) .
.
Note that depending on the
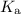 value, the final result might slightly vary.
value, the final result might slightly vary.