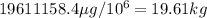Answer:
19.61kg
Step-by-step explanation:
The first thing to do is divide the amount of feet into 3.28:
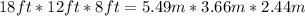
With this information, we calculate the volume:
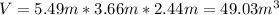
As we have the concentration and the volume, we can calculate the mass of CO:
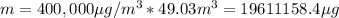
As this is a very big number, we can express it in kg by dividing in 10^6: