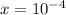Answer:
x=1×10−4
Explanation:
we have
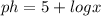 -----> equation A
-----> equation A
The pH of a given solution is the negative log base 10 of the concentration of hydrogen ions, H+
so
ph=-log[H+]
In this problem we have the concentration of hydrogen ions
[H+] =0.1 M
That means that the pH of the solution will be
ph=-log[0.1]
Rewrite
ph=-log[10^-1]
ph=-(-log10)
Remember that
log10=1
substitute
ph=-(-1)
ph=1
substitute the value of ph in equation A and solve for x
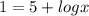
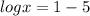
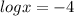
applying property of logarithms