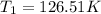Answer: The original temperature was
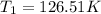
Step-by-step explanation:
Let's put the information in mathematical form:
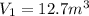
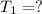
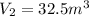
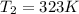
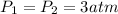
If we consider the helium as an ideal gas, we can use the Ideal Gas Law:

were R is the gas constant. And n is the number of moles (which we don't know yet)
From this, taking
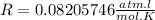 , we have:
, we have:
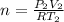
⇒
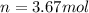
Now:
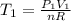
⇒