Answer: Option (C) is the correct answer.
Step-by-step explanation:
As the given reaction is as follows.
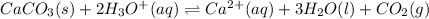
According to Le Chatelier's principle, any disturbance caused in an equilibrium reaction will shift the equilibrium in a direction that will oppose the change.
Hence, in order to favor the formation of products when we remove
 then there will occur decrease in it's concentration.
then there will occur decrease in it's concentration.
As
 is forming on the product side and equilibrium will shift in the direction where there is less stress.
is forming on the product side and equilibrium will shift in the direction where there is less stress.
Hence, then equilibrium will shift in the forward direction, that is, on the products side.
Thus, we can conclude that removing
 as it is formed would force the reaction to favor the products.
as it is formed would force the reaction to favor the products.