Answer: The correct answer is Option E.
Step-by-step explanation:
According to the Bronsted-Lowry conjugate acid-base theory:
An acid is defined as a substance which looses donates protons and thus forming conjugate base.
A base is defined as a substance which accepts protons and thus forming conjugate acid.
To form a conjugate acid of
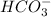 , this compound will accept one proton to form
, this compound will accept one proton to form
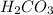
The conjugate acid formed is named as carbonic acid.
Hence, the correct answer is Option E.