Answer:
The oxidizing agent is the Nitric Acid

Step-by-step explanation:
You have the balanced equation:
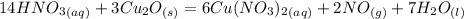
First you should select one by one the reactants and the products and to find their oxidation numbers.
The reactants are on the left of the equation and products are on the right.
So, you have:
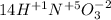
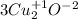
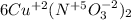
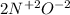
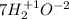
Then, you should find the chemical elements that change its oxidation number, in this case we have:
Cu has an oxidation number of +1 in
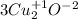 and it changes to +2 in
and it changes to +2 in
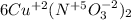 , so Cu loses electrons.
, so Cu loses electrons.
N has an oxidation number of +5 in
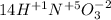 and it changes to +2 in
and it changes to +2 in
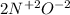 , so N gain electrons.
, so N gain electrons.
So,
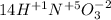 is the oxidizing agent.
is the oxidizing agent.