Answer:
1) 4.0744 grams of aluminum chloride can be obtained.
2) 0.7652 grams of chlorine that react with 0.194 g of aluminum.
Step-by-step explanation:
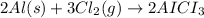
1) Moles of aluminum :
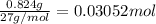
According to reaction, 2 moles of aluminum gives 2 moles of aluminium chloride.
Then 0.03052 mol of aluminum will give with :
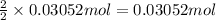 of aluminium chloride.
of aluminium chloride.
Mass of 0.03052 moles of aluminium chloride :
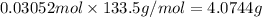
4.0744 grams of aluminum chloride can be obtained.
2) Moles of Aluminium :
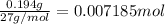
According to reaction, 2 moles of aluminum reacts with 3 moles of chlorine gas.
Then 0.007185 mol of aluminum will react with:
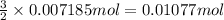 of chlorine gas.
of chlorine gas.
Mass of 0.01077 mol moles of chlorine gas:
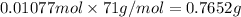
0.7652 grams of chlorine that react with 0.194 g of aluminum.