Answer:
λ = h/p
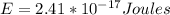
This energy is equivalent to E=150.44eV
Step-by-step explanation:
de Broglie Equation:
λ = h/p
h=6.63×10-34 m2 kg / s Planck's constant
so: p=h/λ
On the other hand, an atomic distance, an angstrom, is equal:
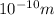 , λ should be similar to this distance.
, λ should be similar to this distance.
Kinetics Energy of a electron, m=9.11×10−31 kg
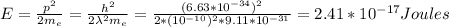
Number of volts needed to accelerate that electron from rest to this energy:
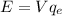
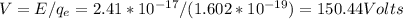
So, this energy is equivalent to E=150.44eV
This high-voltage device is called: particles accelerator