Answer:
h = 23.237 W/m2 K
Step-by-step explanation:
given data:
flow rate = 1.08*10^6 gm/h = 0.3 kg/s
D = 4.5 cm = 0.045 m
L = 10^4 cm = 100 m
surface temperature = 370 K
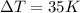
Surface heat of vapor = 2190 J/kg.k
From energy conservation principle we have
heat transfer btwn surface and air = heat loss due to flow and temp. drop
where
heat transfer btwn surface and air is due to convection
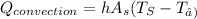
WHERE
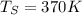
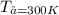
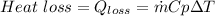
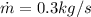
from both above equation we have
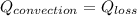
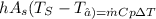
putting all value to get heat transefer coefficient
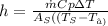
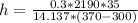
h = 23.237 W/m2 K