Step-by-step explanation:
The given data is as follows.
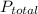 = 1 atm,
= 1 atm,
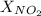 = 0.78
= 0.78
Now, we will calculate the partial pressure of given nitrogen gas as follows.
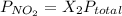
=

= 0.78 atm
According to the Henry's law we will calculate the solubility of nitrogen as follow.
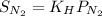
=
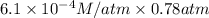
=
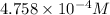
Now, for 99.0 L the amount of nitrogen gas dissolved is calculated as follows.
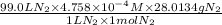
=
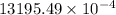
= 1.32 g
Thus, we can conclude that the mass of nitrogen dissolved at room temperature in an 99.0 L home aquarium is 1.32 g.