Answer: The mass of nitrogen monoxide formed is 0.6 grams.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of copper (II) nitrate = 5.58 g
Molar mass of copper (II) nitrate = 187.56 g/mol
Putting values in above equation, we get:
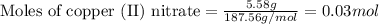
The given chemical equation follows:
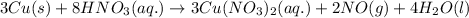
By Stoichiometry of the reaction:
When 3 moles of copper (II) nitrate is formed, then 2 moles of nitrogen monoxide is formed.
So, when 0.03 moles of copper (II) nitrate is formed, then
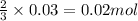 of nitrogen monoxide is formed.
of nitrogen monoxide is formed.
Now, calculating the mass of nitrogen monoxide by using equation 1, we get:
Molar mass of nitrogen monoxide = 30 g/mol
Moles of nitrogen monoxide = 0.02 moles
Putting values in equation 1, we get:
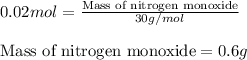
Hence, the mass of nitrogen monoxide formed is 0.6 grams.