Answer:
11.83
Step-by-step explanation:
Ammonia dissolves in water according to the equilibrium expression:
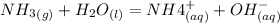
At equilibrium, a small amount dissociates, therefore:
kb = 1.8 * 10⁻⁵
pH = 2.5
the dissociation costant is given by the equilibrium amounts. This is given as:
NH₃ = (2.5 - x)
NH₄ = x
OH = x
The constant, Kb is given by:
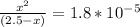
but x is so small that is equal to 0
Hence:
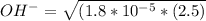
= 0.006708
pOH = -log (OH)
= -log (0.006708)
= 2.173
pH = 14 - pOH
= 14 - 2.173
= 11.826
= 11.83