Answer:
0.359 moles.
Step-by-step explanation:
The oxygen gas has a molecular formula O2. In table periodic, the molar mass of one atom of oxygen is 16 g/mol, so the molar mass of O is 32 g/mol.
Molar mass, mass and number of moles are related by the equation below:
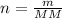
Where n is the number of moles, m is the mass, and MM is the molar mass of the compound.
So, for 11.5 g of O2 produced:
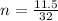
n = 0.359 moles