Answer:
50.08%
Step-by-step explanation:
Hydroxide sodium is a basis, so it's molecule is NaOH (an Arrhenius basis is a cation followed by OH, in this case, sodium is the cation).
The question wants to know the mass percent, so we can choose any calculus basis because the fraction will be the same. So, for convenience, choosing the total number of moles as 1 mol.
The molar fraction of NaOH is 0.3110, so it's the number of moles on the basis is 0.3110, and the rest of the solution is water, which is 0.6890 moles.
The molar mass of NaOH is 40 g/mol (Na = 23 g/mol, O= 16 g/mol, H = 1 g/mol) and the molar mass of water (H2O) is 18 g/mol.
So, calculating the mass by the equation:
m = nxMM
Where m is the mass, n is the number of moles and MM is the molar mass.
NaOH: m = 0.3110x40 = 12.4400 g
H2O: m = 0.6890x18 = 12.4020 g
The total mass of the solution is 24.8420 g, and the mass percent (x) of NaOH is:
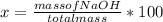
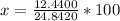
x= 50.08%