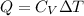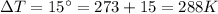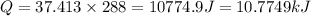Answer:
1. K.E = 11.2239 kJ ≈ 11.224 kJ
2.
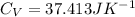
3.
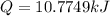
Solution:
Now, the kinetic energy of an ideal gas per mole is given by:
K.E =
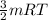
where
m = no. of moles = 3
R = Rydberg's constant = 8.314 J/mol.K
Temperature, T = 300 K
Therefore,
K.E =
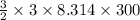
K.E = 11223.9 J = 11.2239 kJ ≈ 11.224 kJ
Now,
The heat capacity at constant volume is:
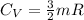
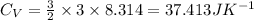
Now,
Required heat transfer to raise the temperature by
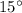 is:
is: