Answer: 34.4 g
Step-by-step explanation:

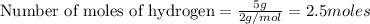
As
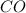 is in excess,
is in excess,
 is the limiting reagent and thus it will limit the formation of products.
is the limiting reagent and thus it will limit the formation of products.
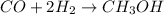
According to stoichiometry:
2 moles of hydrogen produce = 1 mole of
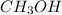
2.5 moles of hydrogen produce =
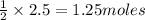 of
of
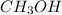
Mass of
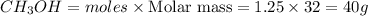
But as % yield is 86%, mass of
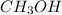 produded is
produded is
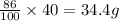
Thus 34.4 g of
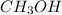 is produced.
is produced.