Answer:
The final temperature of ethane is 323.8°C
Step-by-step explanation:
Given that
At initial condition
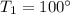
Lets take initial volume of ethane is V,then the final volume of ethane increase to 60% it means the the final volume is 1.6V.
As we know that at constant pressure
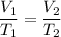
So now by putting the values
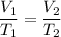
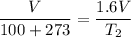
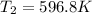
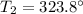
So the final temperature of ethane is 323.8°C