Answer:
The lake's depth is
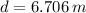
Step-by-step explanation:
To find out how deep the lake is, first we must ask ourselves why the volume of the bubble changes. Let's assume ideal gas behaviour. That is:
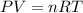
Or we could also put it this way:
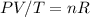
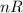 remains constant (we assume that the number of molecules in the gas doesn't change, no gas is absorbed by the water, and no air is stripped from the water) but
remains constant (we assume that the number of molecules in the gas doesn't change, no gas is absorbed by the water, and no air is stripped from the water) but
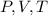 change, they are not the same at the top and at the bottom. V and T change, we know that from the problem's data, but does P change? Yes, it changes because of hydro-static pressure, the deeper we go, the higher the pressure, because of the water column above.
change, they are not the same at the top and at the bottom. V and T change, we know that from the problem's data, but does P change? Yes, it changes because of hydro-static pressure, the deeper we go, the higher the pressure, because of the water column above.
We assume that pressure at the surface is standard atmospheric pressure, 101325 Pa.
Now, we can write:
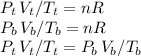
Where b and t stand for bottom and top respectively.
We can solve for
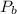 as follows:
as follows:
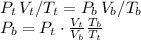
And replacing
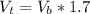 (Because the volume at the top increased 70%) we can calculate
(Because the volume at the top increased 70%) we can calculate
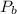
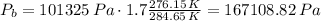
(Note that we use the absolute temperature scale! this is very important)
Now, we know that:
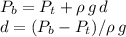
where
 and
and
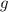 are water's density and gravity's acceleration respectively.
are water's density and gravity's acceleration respectively.
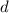 stands for the depth of the lake. Now, water's density changes with temperature, but only slightly in the range of temperatures for this problem (by around 1 in a thousand parts), so we can take the standard value for water density:
stands for the depth of the lake. Now, water's density changes with temperature, but only slightly in the range of temperatures for this problem (by around 1 in a thousand parts), so we can take the standard value for water density:
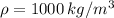
Plugging that into the equation we have:
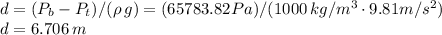
And thus we have found the depth of the lake.