Answer: The average atomic mass of the element X is 51.99592 amu
Step-by-step explanation:
Mass of isotope 1 = 49.94605 amu
% abundance of isotope 1 = 4.350% =
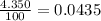
Mass of isotope 2 = 51.94051 amu.
% abundance of isotope 2 = 83.79% =
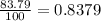
Mass of isotope 3 = 52.94065 amu.
% abundance of isotope 2 = 9.500% =
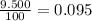
Mass of isotope 4 = 53.93888 amu.
% abundance of isotope 2 = 2.360% =
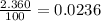
Formula used for average atomic mass of an element :
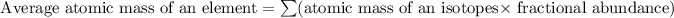
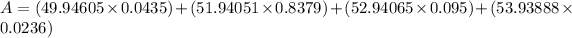
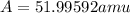
Therefore, the average atomic mass of the element X is 51.99592 amu