Answer:
Amount of sucrose required = 278.85 g
Step-by-step explanation:
(w/v)% = 39.0 %
Volume of the solution = 715 mL
39% (w/v) means 39 g of sucrose present in 100 mL of the solution.
Using unitary method,
For making 39% solution,
100 mL solution requires 39 g of the sucrose
therefore, 715 mL solution requires:
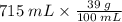
= 278.85 g