Answer:
green light have high energy
Step-by-step explanation:
We have given the wavelength of the red light
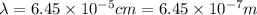
Speed of the light
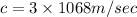
The energy of the signal is given by
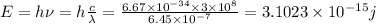
The frequency of the green light is given by:
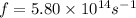
So energy
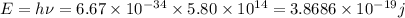
So green light have high energy