Answer:
45.6°C
Step-by-step explanation:
Kinetic energy =
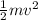
Use m= 1.80kg and v=7.80m/s (mass and speed of hammer).
K = 0,5*1.80kg*(7.80m/s)^2 = K=54.8J
Heat is 60% of Kinetic energy. Q = 0.6*54.8J = 32.9J
As it is stuck 10 times the total heat is 10*32.9J = Total Heat = 329J
Use the equation
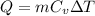 to find change of temperature:
to find change of temperature:
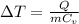
Q = 329J; m = 8.00g of aluminium; C_v = 0.900J/g°C (For aluminium)
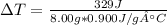
Calculating gives Change of Temperature = 45.6°C