Answer:
The electronic structure for Ca is:
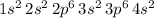
The outer shell electrons are 2:
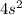
Step-by-step explanation:
To explain the order in which electrons orbitals are filled in this case, we use Madelung's rule, which states that electron orbitals are filled by increasing order of n+l, where n is the principal quantum number and l is the orbital angular momentum number (or azimuthal quantum number).
If there are different orbitals with the same value for n+l, for example n=2 l=1, or n=3 l= 0, the orbital with the lowest n number is filled first. (2p before 3s)
We know that the letters s,p,d,f correspond to azimuthal quantum numbers 0,1,2,3 respectively. s orbitals take only 2 electrons, p orbitals take 6 electrons, and d orbitals take 10 electrons.
Therefore, the order in which orbitals are filled would be:
1s - 2s - 2p - 3s - 3p - 4 s - 3 d - 4 p - 5s and so on.
Ca has only 20 electrons, and one can see that it takes exactly 20 electrons to fill the first 6 orbitals (up to and including 4s)
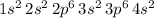
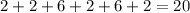
Outer shell electrons are those for which n is greatest, in this case the greatest value for n is 4, and there are only two electrons in the
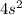 orbital, therefore there are 2 valence electrons.
orbital, therefore there are 2 valence electrons.