Answer : The mass percent of Douglasite is 63.75 %
Explanation :
First we have to calculate the moles of
 .
.
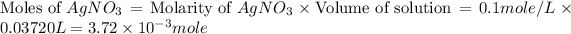
As we know that,
Moles of
 = Moles of AgCl = Moles of
= Moles of AgCl = Moles of
 =
=

The formula of Douglasite is,
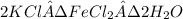
The molar mass of Douglasite = 311.88 g/mole
Now we have to calculate the moles of
 atoms.
atoms.
In the formula of Douglasite, there are 4
 atoms.
atoms.
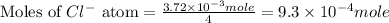
Now we have to calculate the mass of Douglasite.

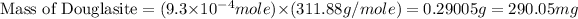
conversion used : (1 g = 1000 mg)
Now we have to calculate the mass percent of Douglasite.
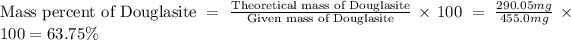
Therefore, the mass percent of Douglasite is 63.75 %