Step-by-step explanation:
As we know that density is the amount of mass present in a liter of solution.
Mathematically, density =

As it is given that density of water is 1 g/ml and volume is 175 mL. So, mass of water will be calculated as follows.
density =

1 g/ml =
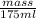
mass = 175 g
It is known that 18 g of water contains 1 mole of water. Hence, number of moles present in 175 g of water will be calculated as follows.
No. of moles =

=
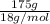
= 9.72 mol
This means that we have 9.72 moles of ethanol also.
Hence, volume of ethanol present in 175 ml of water will be as follows.
No. of moles of ethanol =
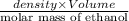
9.72 mol =
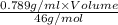
Volume = 566.69 ml
Thus, we can conclude that 566.69 ml volume of ethanol contains the same number of molecules as are present in 175 ml of
 .
.