Answer:
C. The reaction is energetically favorable.
Step-by-step explanation:
The reaction which shows the removal of the terminal phosphate from the ATP is shown below:
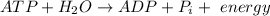
The Gibbs' free energy change of this reaction,
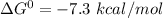
Hence, Option A is not correct.
It is a type of hydrolysis reaction in which water is being added to the molecule.
Hence, Option B is not correct.
The Gibbs' free energy change is negative which means that the reaction is energetically favorable.
Option C is correct.