Answer:
Step-by-step explanation:
Our
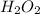 sample yielded 1g of
sample yielded 1g of
 and 16g of
and 16g of
 , but our unknown sample yielded 2 times as much
, but our unknown sample yielded 2 times as much
 for the same amount of
for the same amount of
 .
.
What does this mean? that the H:O proportion for the unknown sample is twice the H:O proportion for the
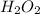 sample.
sample.
What is the H:O proportion for the
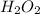 sample? As we can see from its formula, it's 1:1, therefore the proportion for the unknown formula must be 2:1.
sample? As we can see from its formula, it's 1:1, therefore the proportion for the unknown formula must be 2:1.
That means, two H atoms for every O atom. We could write that as:
 and you should recognize that formula, for it is one of the most common compounds on earth, Water.
and you should recognize that formula, for it is one of the most common compounds on earth, Water.